Recently, Dr. Jun Liao at SLST, teamed with research groups of Drs. José D Faraldo-Gómez in NIH and Youxing Jiang at UT Southwestern Medical center, has published a research article on the journal Nature Structural and Molecular Biology (IF=13.309) titled with “Mechanism of extracellular ion exchange and binding-site occlusion in a sodium/calcium exchanger”. Drs. Jun Liao, were one of corresponding and leading authors.
Na+/Ca2+ exchangers (NCX) are widely distributed on plasma membrane of various cells and act as a class of essential secondary active transporters in Ca2+ signaling and its homeostasis. NCX usually couple the ascending expulsion of intracellular Ca2+ across the plasma membrane with the descent translocation of Na+ into the cell. NCX play important physiological roles in the activities such as insulin secretion, the migration and differentiation of gliacytes, the release of neurotransmitters and excitation-contraction coupling of muscle cells. Most notably, NCX were regarded as a central player in the regulation of calcium transient during the cardiac excitation-contraction coupling process. The dysfunction or dysregulation of NCX will lead to heart failure, arrhythmia, and pathophysiological changes to central nervous and endocrine systems.
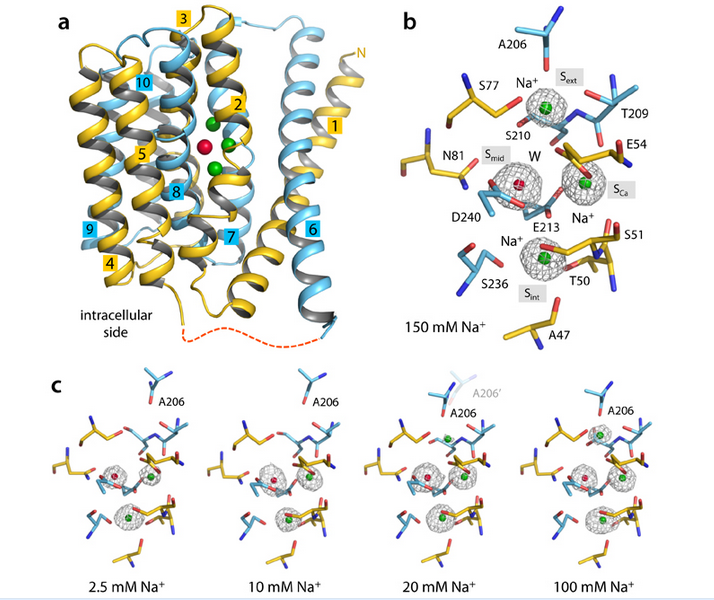
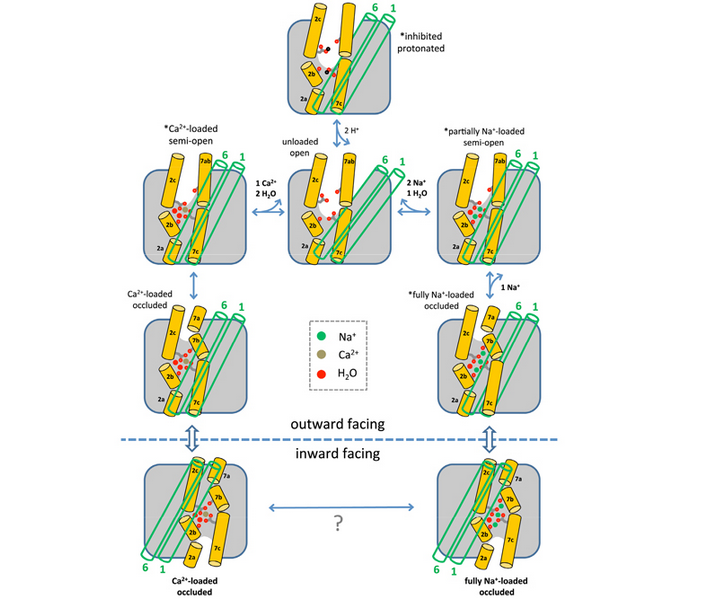
Dr. Liao and his collaborators have concentrated on an archaeal NCX from Methanococcus jannaschii (NCX_Mj), which only consists of the highly conserved ion transduction domain of its eukaryotic counterparts, and thus eliminated extra-disturbance to the ion exchange measurement. The researchers have proposed an antagonistic mechanism of multiple sodium binding to the affinity of calcium at its ion-binding site in their previous work (Science (2012) 335, 686-690). The postulated model explained well of classic 3Na+:1Ca2+ stoichiometry and cooperativity of ion exchange process in NCX. In the current paper, scientists took further analysis on the features of outward-facing NCX_Mj in complex with Na+, Ca2+or Sr2+, and in an empty state. The analysis revealed the nature of the conformational changes associated with the occupancies of Na+, based on the binding manner and comparative affinity of above cations. The authors also corroborated the structural foundation for the flexible exchange stoichiometry from 2Na+:1Ca2+ to 4Na+:1Ca2+ besides the typical 3:1 deduced from functional studies. The independent molecular-dynamics calculations forecasted similar results, based on the conformational free-energy landscape of the transporter in different ion-occupancy states.
Functional studies on NCX have revealed many features of ion exchange since the discovery of the antiporter on 1968. One of them is the “two-site” model: a divalent site binds either 1 Ca2+ or 1-2 Na+ ions while a second site bound by at least one additional Na+; 3 Na+ are leastwise essential at the extracellular side to fetch Ca2+ movement. The model is now readily interpreted at atomic level by the current article.
Journal Reference:
Liao J*#, Marinelli F*, Lee C, Huang Y, Faraldo-Gómez JD#, Jiang Y# (*, first author; #, correspondence) (2016) Mechanism of extracellular ion exchange and binding-site occlusion in a sodium/calcium exchanger, Nat. Struct. Mol. Biol.23,590-599