SLST Professor Shen Wei’s lab, in collaboration with Huazhong University of Science & Technology Professor Li Haohong’s Lab, have discovered a novel mechanism of predatory hunting and reward feeding. Their research, published on May 23rd in an article in Nature Neuroscience entitled “Zona incerta GABAergic neurons integrate prey-related sensory signals and induce an appetitive drive to promote hunting” shows that feeding and hunting are crucial for animal survival and are the driving force for evolution, hot topics in neuroscience research. These behaviors involve feature detection of prey, motivation encoding and also are important for energy homeostasis. Analyzing these mechanisms might be helpful to treat obesity, anorexia and motivation dysfunctions. The research team utilized advanced technologies such as optogenetics, in vivo electrophysiology and calcium fiber photometry to show that the zona incerta (ZI) integrates prey-related multisensory signals and induces a motivational drive to guide prey detection and promote predatory attack.
They found optogenetic activation of THE ZI induced binge eating, undirected gnawed food containers and breeding materials. These behaviors are reminiscent of predatory hunting behaviors that require biting attack. Therefore, they hypothesized that THE ZI plays a critical role in predatory hunting. To test this, they introduced crickets to mice and found ZI activation remarkably increased pursuit speed, bite attacks, and hunting success rate. These induced attacks were only observed when introducing live prey (crickets) and artificial prey (electric insects), but not intraspecific counterparts, suggesting that ZI GABAergic neurons play a role in predatory hunting. They also found optogenetic inhibition of ZI GABAergic neurons abolished prey attack and eating. Furthermore, genetic deletion of GABA function strongly impaired hunting and reduced high-fat intake but not chow intake. Together, these findings demonstrated that both ZI GABAergic neurons and GABA are required for efficient predatory hunting and reward feeding.
How were prey-derived feature signals detected? They hypothesized ZI neurons might be sensitive to hunting-related signals. Using in vivo multi-channel recording, they found crickets, food pellets and textured objects activated different subsets of ZI neurons. Then, they showed that ZI neurons were activated by multiple prey-related sensory stimuli, such as whisker somatosensory, moving visual dots and auditory signals. Surprisingly, these sensory sensitive neurons largely and mutually overlapped, suggesting a sensory integration mechanism.
Does THE ZI induce strong motivation to drive hunting and feeding? Through real-time place preference assays, they found that mice had a strong preference to the laser-stimulated side that coupled with neural activation, suggesting a positive valence. In operant tasks such as intracranial self-stimulation, ZI activation led to strong repetitive and progressive self-stimulatory nose poking in order to receive a ‘laser’ reward, which established that THE ZI induces a strong appetitive motivational drive.
Furthermore, they showed that ZI GABAergic projections to the midbrain periaqueductal gray (PAG) promoted hunting and encoded an appetitive motivation, while ZI GABAergic neurons projected to the paraventricular thalamus (PVT) to promote feeding (also see Zhang X, van den Pol A N. Science, 2017). Together, Shen and Li groups delineated the function of ZI GABAergic neurons in hunting, which integrates prey-related sensory signals into prey detection and attack, and induces a strong appetitive motivational drive. The identification of THE ZI as a reinforcing center may also provide an explanation for the altered pleasure-seeking behaviors observed after subthalamic deep-brain stimulation near ZI.
Dr. Shen Wei (ShanghaiTech University) and Dr. Li Haohong (Huazhong University of Science and Technology) are the corresponding authors of the article. This study was supported by Molecular Imaging Core Facility (MICF) and the Molecular Cellular Core, ShanghaiTech University. The research was funded by the National Natural Science Foundation of China and ShanghaiTech Start-up Fund.
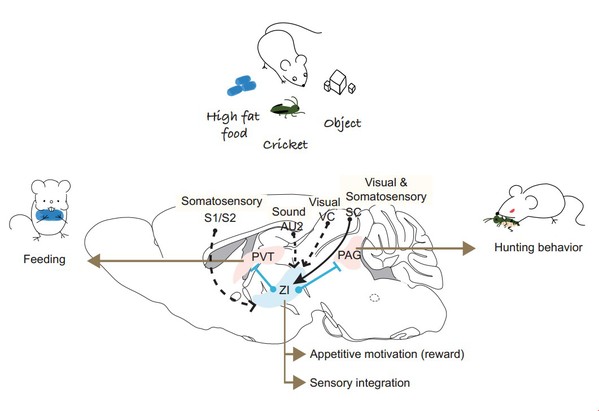
Figure 1. Function of Zona Incerta GABAergic neurons in hunting and feeding. Zona Incerta GABAergic neurons integrate prey-related sensory signals and induce an appetitive drive to promote hunting. AU2, secondary auditory cortex; S1, primary somatosensory cortex; S2, secondary somatosensory cortex; VC, visual cortex; SC, superior colliculus; PAG, periaqueductal gray matter; PVT, paraventricular thalamic nucleus; ZI, zona incerta.


