On April 22, the international journal Development published online a paper about the mechanism of brain development entitled “Heterogeneous nuclear ribonucleoprotein A3a controls mitotic progression of neural progenitors via interaction with cohesin”. This study as carried out by the group of Dr. Zhen-Ge Luo at the School of Life Science and Technology, ShanghaiTech University, in collaboration with Dr. Qiang Sun at the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences.
The cerebral cortex of mammals controls sensory input, motor output, learning and memory, as well as decision-making and cognition. During neocortical development, distinct subtypes of excitatory neurons are born in well-ordered temporal patterns via direct or indirect neurogenesis pathways and migrate to form multiple layers with ‘inside out’ manner to form deep and up layers sequentially. Radial glia cells (RGs) localized in apical ventricular zone (VZ) can undergo either symmetric proliferative division, generating two daughter RGs and expanding the progenitor pool, or asymmetric neurogenic division to produce a RG cell and a postmitotic neuron or an intermediate progenitor (IP) residing in the subvetricular zone (SVZ). Nevertheless, molecule mechanisms that tightly control successive NP division and temporal progression of cortical neurogenesis are not fully understood.
In this study, they report a role of heterogeneous nuclear ribonucleoprotein A3 (HNRNPA3) in regulating the division of early cortical NPs that mainly give rise to deep-layer neurons via direct neurogenesis. HNRNPA3 is highly expressed in NPs of mouse and human cortex at early stages with a unique peri-chromosome pattern. Intriguingly, down-regulation of HNRNPA3 caused chromosome disarrangement, which hindered normal separation of chromosomes during NP division, leading to mitotic delay. Furthermore, HNRNPA3 is associated with the cohesin-core subunit SMC1A and controls its association with chromosomes, implicating a mechanism for the role of HNRNPA3 in regulating chromosome segregation in dividing NPs. Hnrnpa3 deficient mice exhibited reduced cortical thickness, especially of deep layers. Moreover, down-regulation of HNRNPA3 in cultured human cerebral organoids led to marked reduction in NPs and deep-layer neurons. Thus, this study has identified a critical role of HNRNPA3 in NP division and highlighted the relationship between mitosis progression and early neurogenesis.
The first author of this paper is Min-Yi Ou, a Ph.D student supervised by Dr. Zhen-Ge Luo. This study was partially supported by grants from National Natural Science Foundation of China, the Frontier Key Project of the Chinese Academy of Sciences, Shanghai Municipal Science and Technology Major Project, and start-up support by ShanghaiTech University.
Read more at: https://dev.biologists.org/content/early/2020/04/21/dev.185132
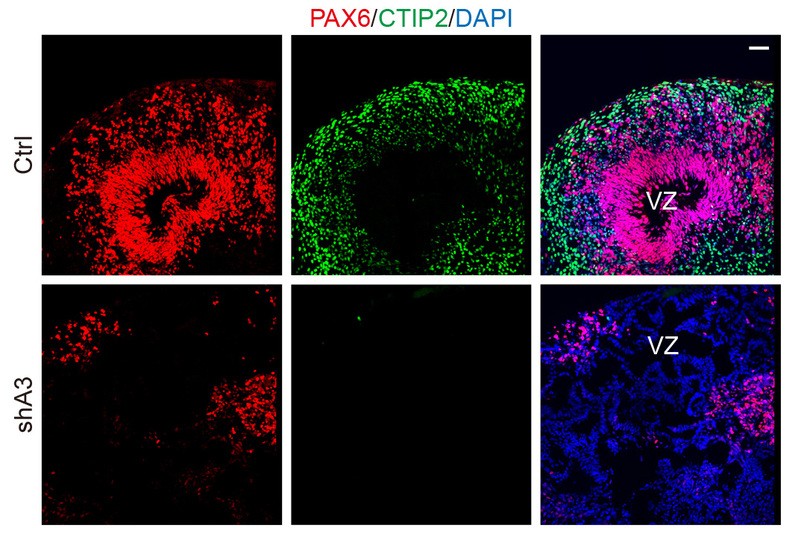
HNRNPA3 down-regulation affects neurogenesis of cultured human cerebral organoids


