Recently, significant breakthroughs have been made in cancer immunotherapy. Immunologists have successfully cured cancer patients by using biologic (e.g., checkpoint blockade) and cellular drugs (e.g., TCR-T and CAR-T) to enhance the anti-cancer ability of patients' own T cells. The major goal of our laboratory is to explore the regulatory mechanism of T cell anti-tumor ability and develop new cancer immunotherapies by combining proteomic, CRISPR screen, and mouse genetics.
Engineered T cell therapies, mainly CAR-T and TCR-T, have become the new frontier of cancer treatment. CAR-T and TCR-T therapies differ in many aspects, including cell persistence and toxicity, leading to different therapeutic outcomes. Both TCR and CAR recognize antigens and trigger T-cell mediated antitumor response, but they have distinct molecular structures and signaling properties.
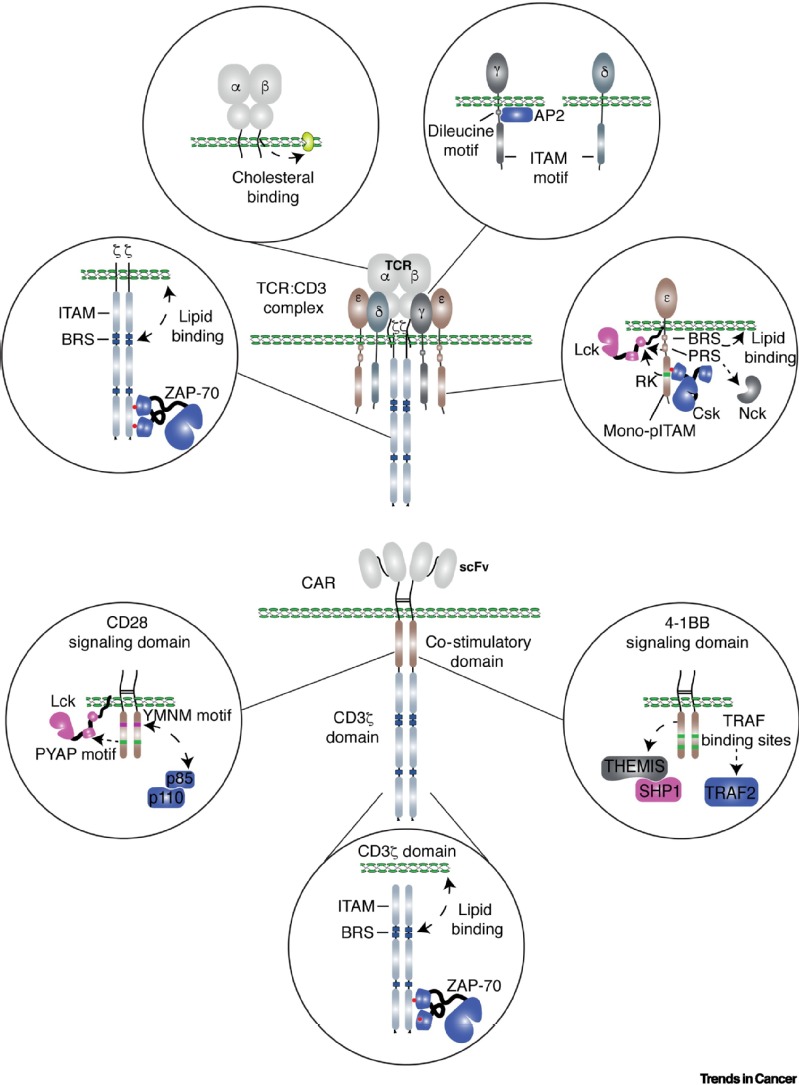
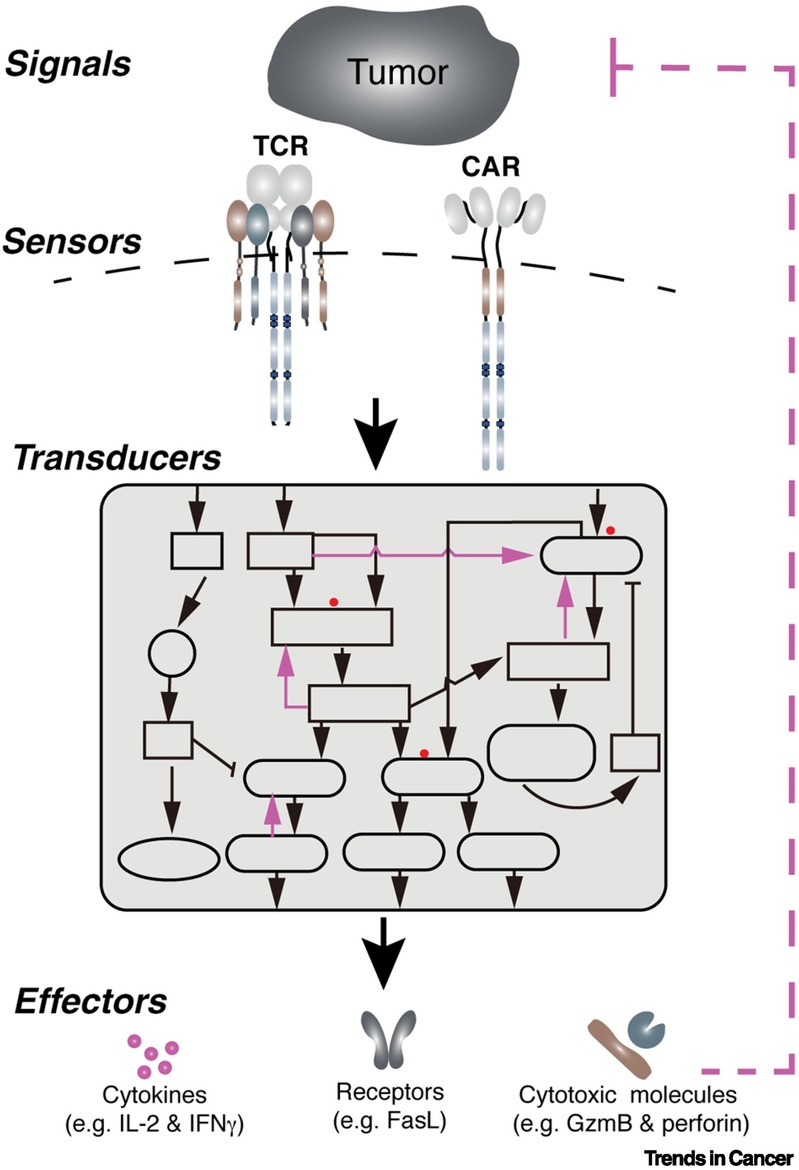
The antitumor response in the engineered T cells can be conceptually divided into three stages: i. The sensors on the T cell membrane, e.g., TCR and CAR, scan the surrounding environment and recognize tumor antigens on tumor cells; ii. The information is transduced and amplified through a signal transduction network to activate T cells; iii. An array of effector molecules are activated or produced to execute antitumor responses (see the Figure below and our review published in Trends in Cancer, 2021). The sensors, transducers, and effectors are the essential components of the antitumor response of engineered T cells. Positive and negative feedback loops are also involved in enabling these three components to induce a potent and durable antitumor response. TCR-T and CAR-T exhibit several distinct clinical features, including in vivo persistence of transferred T cells, the number of T cells transferred to the patients and the efficacy against solid tumors. These differences are contributed, at least partially, by the distinct features of sensors and transducers of TCR-T and CAR-T cells. Understanding how engineered T cells sense the tumor antigens and how signaling networks control T cell antitumor activities will help us develop next-generation engineered T cell therapy. Collectively, in my eyes, the primary function of the egineered T cells is to serve as an information processor, where inputs are recongized and sorted,followed by the activated effector mechanisms. Thus, our laboratory is focusing on the optimizing the sensors and transducers, as well as the effector molecules, that regulate T cell antitumor function.