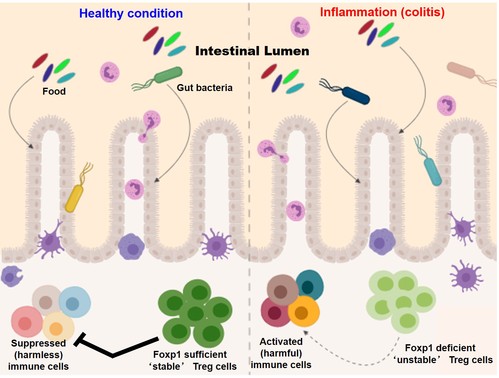
While there is reasonable success in using ex vivo expanded natural Treg (nTreg) cells in clinical settings, efforts are ongoing for employing in TGF-β mediated in vitro induced Treg (iTreg) cells for adoptive transfer therapy. In that regard, we have identified the transcription factor Foxp1(please note, it is a member of the same fork-head transcription factor family in which Foxp3 belongs; so in a way it is a 'molecular sibling' of Foxp3), to provide stability and identity specifically to iTreg cells. Interestingly Foxp1 appeared to be dispensable as far as maintaining the expression of Foxp3 in thymically derived tTreg population is concerned. Foxp1deletion from established iTreg cells resulted in dramatic instability of the expression of Foxp3. Furthermore, Treg specific deletion of Foxp1 resulted inseverely reduced iTreg generation in mice, and in significantly enhanced intestinal inflammation. These results significantly enhance our understanding of the molecular events contributing to basic aspects of immune cell differentiation processes. Furthermore, in the context of immunotherapy these findings offer an interesting target that can stabilize and expand clinically feasible iTreg cells in clinical settings, thereby potentiating therapeutic possibilities.
Article Reference: https://www.nature.com/articles/s41467-018-07018-y
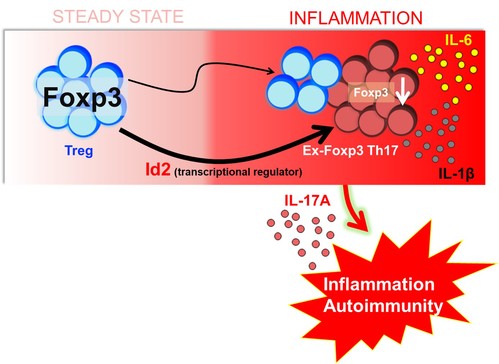
Although role of Treg cells in maintaining immune homeostasis is well established, under inflammatory conditions, Treg cells are known to convert into pathogenic IL-17 producing cells, contributing to the pathogenicity of autoimmune diseases. These Treg cells that have lost Foxp3 and become TH17 cells are referred to as “ex-Foxp3 TH17” cells. While investigating the mechanism of generation of these cells, in collaboration with Dr. Sin-Hyeog Im’s laboratory at POSTECH, we have reported that Id2, a transcriptional regulator expressed in “ex-Foxp3 TH17” cells at a significantly higher level than Treg cells, triggers plasticity in Treg cells and consequently results in enhanced autoimmunity in mice. Cellular fate mapping experiments revealed enhanced Treg plasticity, resulting in exacerbated experimental autoimmune encephalomyelitis (EAE) pathogenesis as well as enhanced anti-tumor immunity. These findings strongly imply that efficiently controlling Id2 activity may provide a novel approach for effective Treg cell immunotherapies in both autoimmunity and cancer, thereby underscoring the robust therapeutic applicability of this study.
Article reference: https://www.nature.com/articles/s41467-018-07254-2
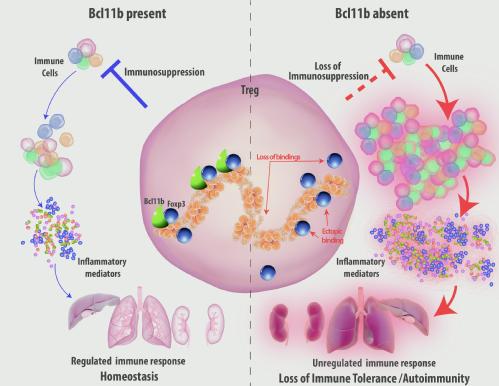
Bcl11b is a DNA binding protein like Foxp3, which caught our eyes since we identified it as a strong physically interacting partner of Foxp3. Just as our friends shape who we are, in the molecular world, the interacting partners of a protein are known to determine its function. Therefore to determine Bcl11b’s role in the function of Treg cells, we generated a mouse where Bcl11b is specifically depleted from Treg cells. Incredibly, this resulted in systemic autoimmunity in mice, which was characterized by inflamed spleen and lymph nodes, cytokine storm, increased antibody titer, as well as overt activation of the immune system. Consequently these mice were moribund at a very early age of their life. This was reminiscent of mice that completely lacked functional Treg cell population. Only difference being, in these excessively sick and autoimmune mice we could still see nearly equal numbers of Treg cells with Foxp3 in them, indicating that Treg cells in addition to Foxp3 critically require Bcl11b in order to restrain systemic autoimmunity. Upon performing further experiments, the mechanism by which Bcl11b functions, unfolded. We unraveled three major functions of Bcl11b in Treg cells. First, it is an essential cofactor of Foxp3 that is required to switch on genes that shape Treg cell identity. Second, Bcl11b is essential for guiding Foxp3 to its functional binding sites in Treg cells. More interestingly we found that in the absence of Bcl11b, Foxp3 is misguided and erroneously binds to sites where it is not supposed to bind. This results in erroneous and presumably counterproductive expression of genes that eventually oppose the function of Treg cells. Therefore in our effort to understand basic aspects of the molecular determinants for Foxp3’s function we identify Bcl11b, which unexpectedly turns out to be a molecular guide shaping up the road map for Foxp3 in the milieu of Treg cell genetic architecture. Bcl11b cooperates with Foxp3 in establishing the Foxp3-dependent transcription program in Treg cells. By acting as a molecular guide, it facilitates Foxp3’s efficient recruitment to its relevant binding sites. Deficiency of Bcl11b in Treg cells lead to loss of Foxp3 binding from a large proportion of its bound sites, as well as arbitrary and ectopic binding to sites where it is not supposed to bind. This results in erroneous switching on or off of genes and loss of Treg function. Consequently these events result in complete crash down of immune regulation and lead to systemic whole body autoimmune disease.
In the future, strengthening the interaction between Foxp3 and Bcl11b by molecular intervention, or disrupting their interaction within tumors may prove to be of immense therapeutic significance in autoimmunity and cancer respectively.
Article reference: https://www.science.org/doi/10.1126/sciadv.aaw0706
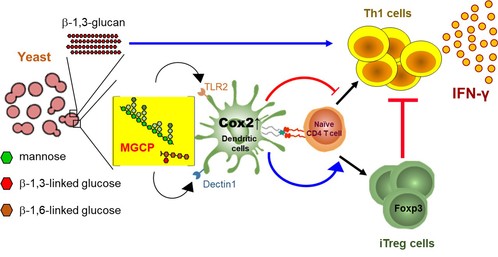
Cell surface polysaccharides derived from microbial species possess multiple immune-modulatory properties, and have the potential to be harnessed to boost anti-tumor immunity or limit overt immune activation. While much work has been done with bacterial species residing at our intestine, functional relevance of individual cell surface polysaccharides of yeast and their effect on the immune system remain largely unknown. In a collaborative study in conjugation with Dr. Sin-Hyeog Im's laboratory at POSTECH, we investigated the immunological properties of commensal yeast species that reside in our intestine, hoping that such knowledge could be potentially harnessed to develop novel drug candidates against inflammatory diseases. We found that independent polysaccharide components of yeast cell wall have pro-inflammatory as well as immune-regulatory properties that otherwise coexist, unless unmasked, upon enzymatic depletion of the other. While β-1,3-glucan, a dominant polysaccharide of yeast cell wall, have immune stimulatory potential that enhance anti-tumor immunity through induction of Th1 differentiation, a relatively minor group of polysaccharides that we termed mannan/β-glucan containing polysaccharides (MGCP), restrain deleterious inflammatory responses through facilitating iTreg cell differentiation as well as repressing IFN-γ production from effector CD4 T cells. MGCP exerts its effect through upregulation of Cox2 in dendritic cells through context dependent Dectin2 or TLR2 mediated pathways.
Together these results not only enhance our understanding of fundamental aspects of the complex interplay between microbiome composition and immune response in host, it also identifies a class of naturally occurring yeast derived cell surface components that can be potentially harvested to treat inflammatory disorders.
Article reference: https://www.nature.com/articles/s41467-021-23929-9
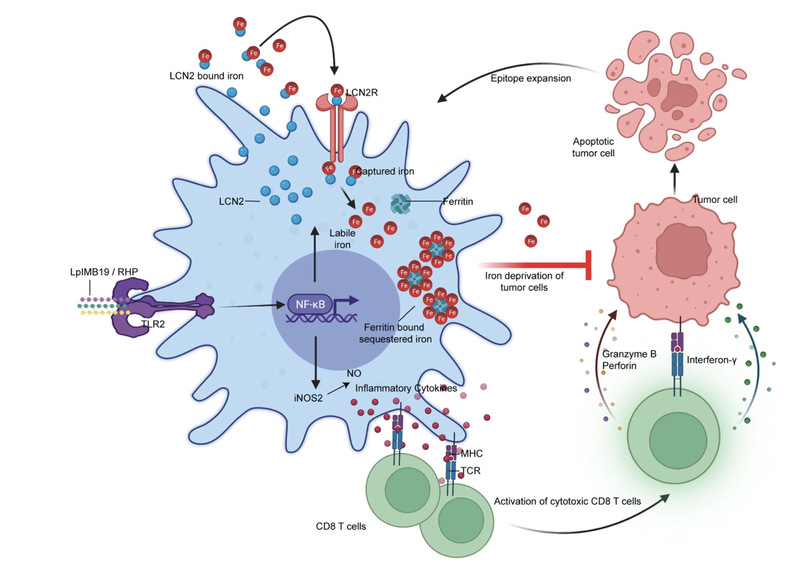
In a recent therapeutically relevant study related to Cancer Immunology, we aimed to identify anticancer properties in commensal organisms present in fermented Korean food, Kimchi. Upon extensive screening of a Kimchi derived library of microbial species, we identified a strain that we named Lactiplantibacillus plantarum IMB19(LplMB19). LpIMB19, we found has enormous potential to trigger antitumor immunity in the context of varied experimental models of cancer. What is more surprising, it does so, by triggering a phenotypic switch in macrophages, which by deploying a high affinity iron sequestration mechanism, starves the tumor cells from iron uptake necessary for survival. A strategy dubbed as nutritional immunity!
Article reference: https://www.nature.com/articles/s41590-024-01816-x
Here is a commentary in the same issue of the journal that describes our work:
https://www.nature.com/articles/s41590-024-01806-z
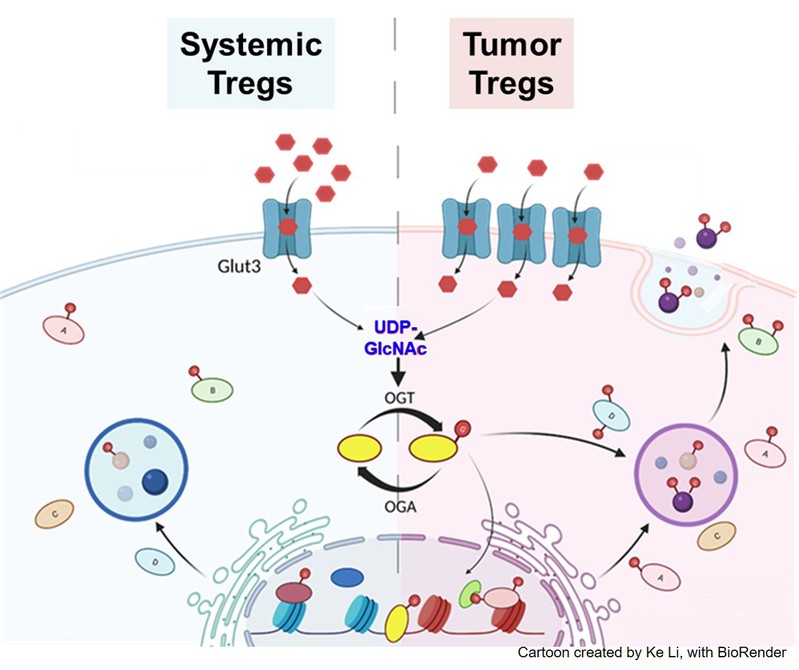
In our attempt to understand tumor-specific functionalities of tumor infiltrating Treg (TIL-Treg) cells, we identified the facilitative glucose transporter Glut3 to be a promising candidate, and therefore generated Treg-specific Glut3 deficient mice. Cellular, metabolic and transcriptomic characterization of these mice implicate Glut3-dependent altered O-linked N-Acetylglucosamine mediated post-translational modification (O-GlcNAcylation) of cellular proteins to be an important determinant of TIL-Treg functionalities. O-GlcNAcylation of proteins is an outcome of an altered glucose utilization pathway, where a small but significant amount of cellular intake of glucose, through the so called hexosamine biosynthetic pathway (HBP), produces the downstream metabolite UDP-GlcNAc. Two key enzymes, the O-GlcNAc transferase (OGT), and O-GlcNAcase (OGA) are responsible to add or remove the post translational O-GlcNAc moiety on cellular proteins respectively. In recent years, dynamically modulated O-GlcNAcylation of various functionally relevant factors, consequently through altered functionalities, have been implicated in human cancers. Our findings indicate that Glut3 mediated glucose transport in TIL-Tregs leads to preferential downstream O-GlcNAcylation of factors, resulting in establishment of a TIL-Treg genetic landscape and gene expression signature with tumor specific functional relevance. Understanding such mechanisms, will be immensely beneficial in the context of identifying distinctive features of tumor infiltrating Tregs that can potentially be utilized in the future as molecular targets for Treg aiming anti-cancer therapies.
Article reference: https://www.nature.com/articles/s41423-024-01229-8


