|
T Cell Signaling and Engineering
Principal investigatorName: Haopeng WangAssociate Professor , PhD, Associate Professor
Position: Affiliation: School of Life Science and Technology
Honor: Education Background:
Working Experience:
Group Introduction Research Area:
Cancer immunity, CAR-T, TCR-T, Immune checkpoints
Research Interests:
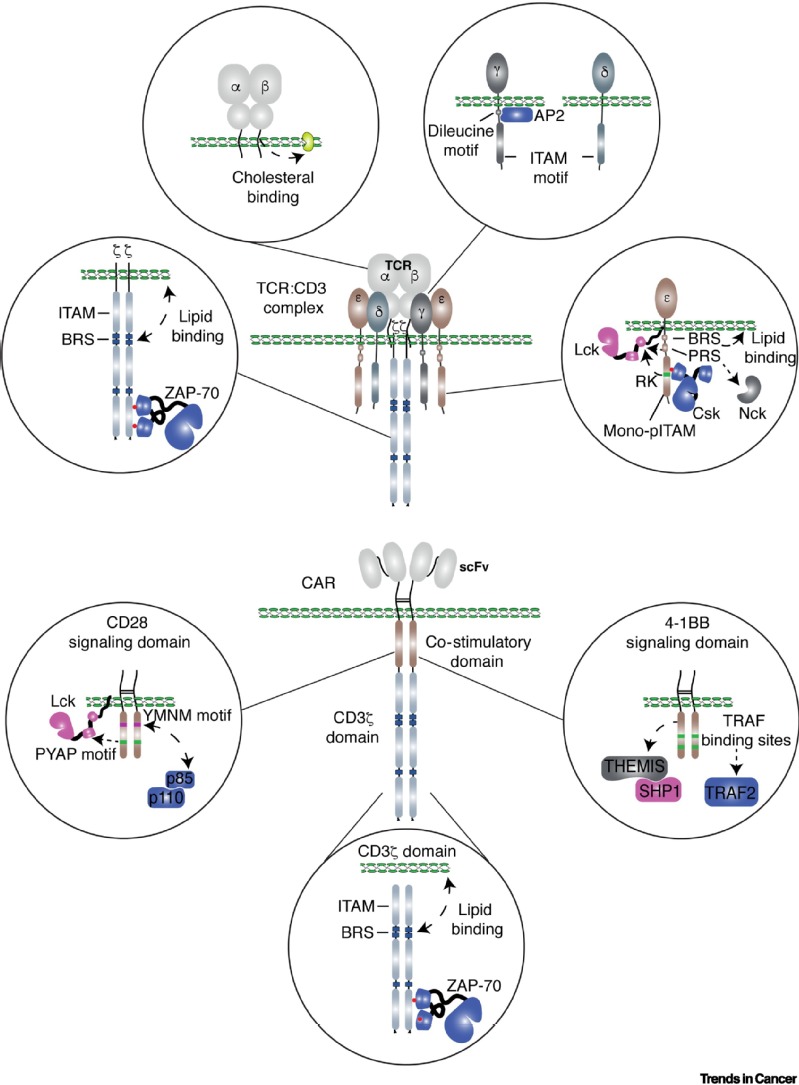
Dr. Haopeng Wang is an Associate Professor and Assistant Dean of the School of Life Science and Technology at ShanghaiTech University. With over 20 years of experience, Dr. Wang's research focuses on T cell signal transduction and engineering. His primary interest lies in unraveling the mechanisms by which natural antigen receptors (e.g., TCR) and synthetic antigen receptors (e.g., CAR) initiate signal transduction events that regulate T cell function in antitumor immunity. By gaining insights into how engineered T cells perceive tumor antigens and how signaling networks govern T cell-mediated antitumor activities, Dr. Wang aims to contribute to the development of next-generation engineered T cell therapy. Recently, his study has made significant progress in elucidating the mechanism underlying CAR tonic signaling and decoding its role in controlling CAR-T therapy efficacy. Recently, significant breakthroughs have been made in cancer immunotherapy. Immunologists have successfully cured cancer patients by using biologic (e.g., checkpoint blockade) and cellular drugs (e.g., TCR-T and CAR-T) to enhance the anti-cancer ability of patients' own T cells. The major goal of our laboratory is to explore the regulatory mechanism of T cell anti-tumor ability and develop new cancer immunotherapies by combining proteomic, CRISPR screen, and mouse genetics. Engineered T cell therapies, mainly CAR-T and TCR-T, have become the new frontier of cancer treatment. CAR-T and TCR-T therapies differ in many aspects, including cell persistence and toxicity, leading to different therapeutic outcomes. Both TCR and CAR recognize antigens and trigger T-cell mediated antitumor response, but they have distinct molecular structures and signaling properties.
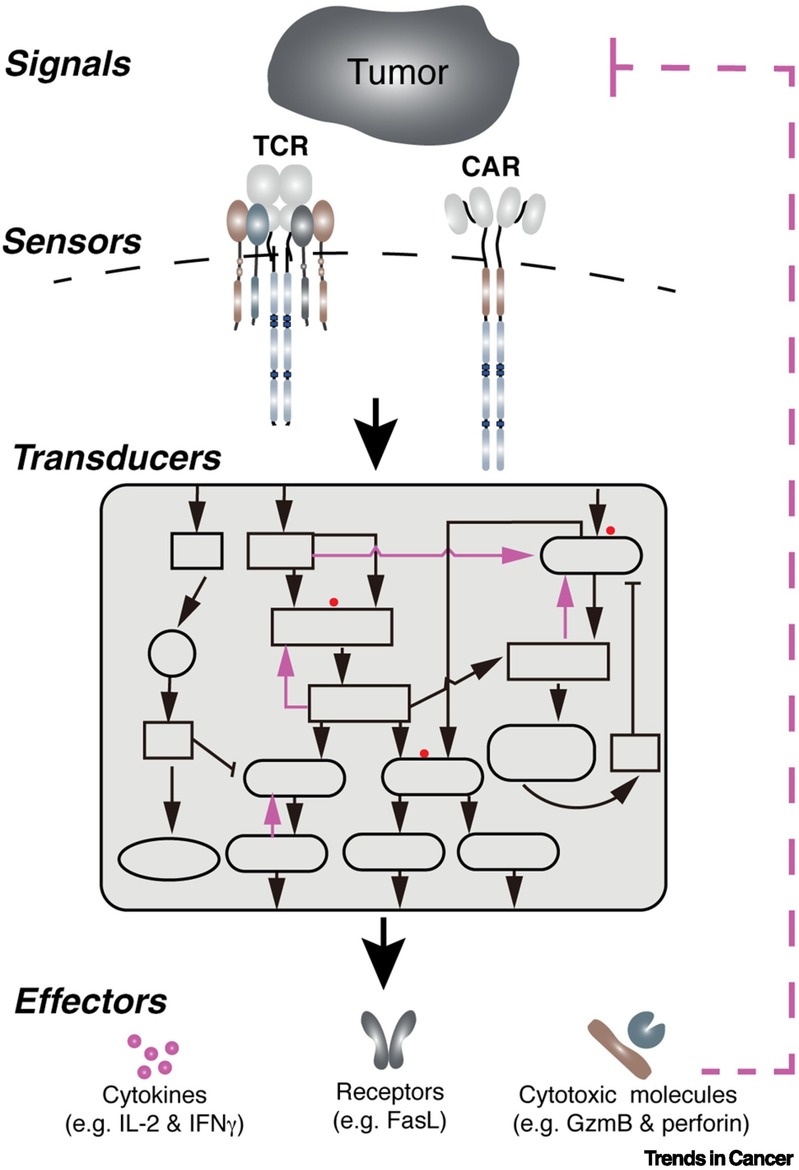
The antitumor response in the engineered T cells can be conceptually divided into three stages: i. The sensors on the T cell membrane, e.g., TCR and CAR, scan the surrounding environment and recognize tumor antigens on tumor cells; ii. The information is transduced and amplified through a signal transduction network to activate T cells; iii. An array of effector molecules are activated or produced to execute antitumor responses (see the Figure below and our review published in Trends in Cancer, 2021). The sensors, transducers, and effectors are the essential components of the antitumor response of engineered T cells. Positive and negative feedback loops are also involved in enabling these three components to induce a potent and durable antitumor response. TCR-T and CAR-T exhibit several distinct clinical features, including in vivo persistence of transferred T cells, the number of T cells transferred to the patients and the efficacy against solid tumors. These differences are contributed, at least partially, by the distinct features of sensors and transducers of TCR-T and CAR-T cells. Understanding how engineered T cells sense the tumor antigens and how signaling networks control T cell antitumor activities will help us develop next-generation engineered T cell therapy. Collectively, in my eyes, the primary function of the egineered T cells is to serve as an information processor, where inputs are recongized and sorted,followed by the activated effector mechanisms. Thus, our laboratory is focusing on the optimizing the sensors and transducers, as well as the effector molecules, to develop next-generation engineered T cell therapy
Research Achievement1. Dissect the mechanism underlying CAR tonic signal and develop a novel CAR-T therapy to prevent T cell exhaustion (Cell Research 2023) CAR-T cell therapy is a kind of tumor immune cell therapy in which the patient's T cells are genetically modified with an artificial receptor, CAR that can recognize tumor cells to achieve specific killing of tumor cells. Many CAR-T therapies have shown promising efficacy in treating hematological tumors but have shown poor efficacy in dealing with solid tumors. One of the crucial reasons is that CAR-T cells will spontaneously produce a continuous basal signal (tonic signaling), which leads to the exhaustion of CAR-T cells and reduces the tumor-killing ability. How does tonic signaling occur, and how does it affect CAR- T efficacy? These scientific questions have been a hot topic in the field.
Our lab, together with Wu Haitao's team at Fudan University, Xu Chenqi's lab at Chinese Academy of Sciences, and Song Xianmin's group at Shanghai 1st General Hospital, published a research paper in the leading international journal Cell Research, successfully dissecting the mechanism of tonic signal formation and proposing a new CAR design strategy. The exhaustion of the resulting novel CAR-T was significantly alleviated, and the anti-tumor efficacy in solid tumors was improved.
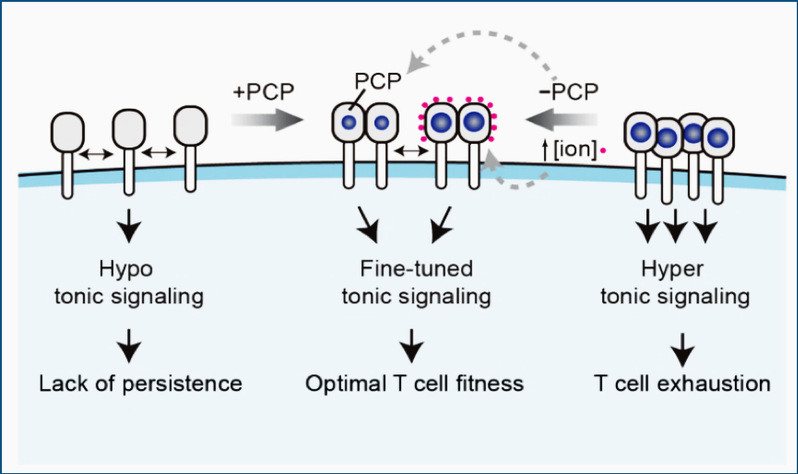
The research team found that electrostatic interactions mediated by positively charged patches (PCP) on the CAR surface drive tonic signal formation. Most CAR-T cells targeting solid tumors tend to be exhausted due to high tonic signal intensity, such as GD2-CAR for glioma and CSPG4-CAR for head and neck tumors. And the tonic signal of such CAR-T cells can be reduced by increasing the ionic strength (salt concentration) in the culture medium or shrinking the PCP on the CAR surface, significantly improving their anti-tumor capacity.
This study has significant implications for the clinical translation of CAR-T therapies. CAR-T designers can use the tonic signal intensity as a design indicator to rationally optimize the structure of CAR, thereby greatly accelerating the CAR-T therapies in the solid tumors field.
2. Develop new CAR-T therapies using synthetic immunology approaches (Immunity 2020, Cover article) CAR-T therapy is a type of cancer immunotherapy that recognizes and kills tumor cells in vivo by expressing chimeric antigen receptors (Chimeric Antigen Receptor, CAR) on a patient's own T cells. CAR-T therapy has shown remarkable efficacy in hematological cancers and is approved for the treatment of acute B lymphocytic leukemia and certain types of non-Hodgkin's lymphoma. However, as a new treatment, CAR-T therapy also has its limitations. More and more clinical data shows that up to 30%-50% of patients treated with CAR-T therapy might experience tumor recurrence, and most of these patients will experience recurrence within one year after receiving CAR-T therapy. There are several reasons for tumor recurrence, of which the in vivo persistence of CAR-T cells (the length of time that CAR-T cells survive in the patient) is a decisive factor. In clinical studies of acute lymphocytic leukemia and chronic lymphocytic leukemia, it has been found that the efficacy of CAR-T therapy is positively correlated with the in vivo persistence of CAR-T cells. Therefore, exploring the regulation mechanism of the in vivo persistence of CAR-T cells has become a current research hotspot. Our team and collaborators published a research paper titled Chimeric Antigen Receptor Designed to Prevent Ubiquitination and Downregulation Showed Durable Anti-tumor Efficacy on Immunity (Cover article). By studying the regulation mechanism of CAR-receptor trafficking in CAR-T cells, a new type of recyclable CAR was designed, which significantly improved the in vivo persistence and anti-tumor effect of CAR-T cells, providing a new strategy for preventing tumor recurrence after CAR T therapy. The technology transfer was successfully completed at the end of 2020 and clinical trials related to recyclable CAR-T are expected to be launched in 2021.
Immunity invited Prof. Stephen Gottschalk and Prof. Hongbo Chi to write a preview for our article.
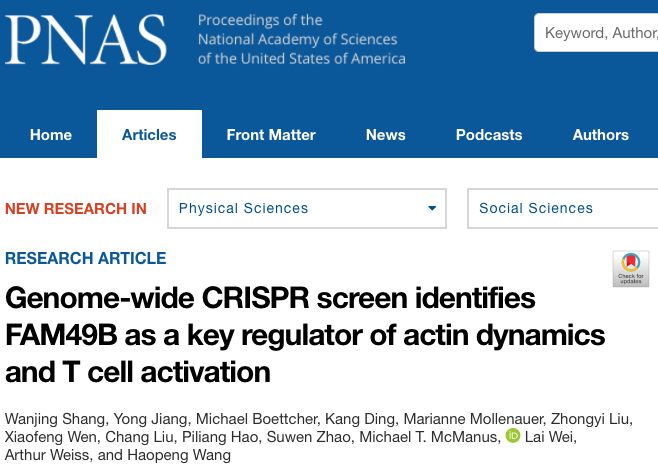
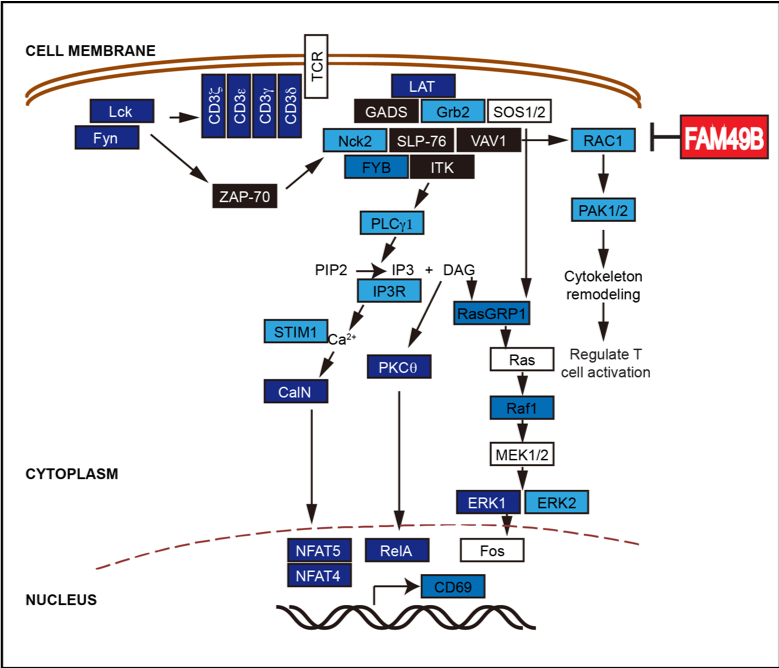
3. Apply a genome-wide CRISPR genetic screen to map T cell functional regulatory networks (PNAS,2018) As an essential part of the human immune system, T cells play a vital role in immune monitoring and killing tumor cells. However, tumor cells can inhibit the anti-tumor activity of T cells via multiple mechanisms, thereby escaping from the body's immune system attack. Therefore, tumors can be treated by increasing the activity of T cells in the clinic. At present, T cell-based tumor immunotherapy has achieved remarkable success in clinical practice and has broad application prospects. However, the existing treatments are only effective for a fraction of cancer patients, and there are considerable side effects. Therefore, further investigation on the regulation mechanism of T cell activation will contribute to developing new tumor immunotherapies to improve the efficacy and benefit more patients. In cooperation with Arthur Weiss lab at UCSF and Lai Wei's group at Sun Yat-sen University, our research team published a research paper titled A genome-wide CRISPR screen identifies FAM49B as a key regulator of actin dynamics and T cell activation in PNAS. In the previous studies from our laboratory, a gene-editing toolbox for studying T cells has been established. This project used a genome-wide CRISPR genetic screen to systematically study the molecular mechanism of T cell activation and illustrate a regulatory map of human T cell functions. It is worth mentioning that this study is the FIRST reported genome-wide CRISPR screen on T cells.
Dr. Lawerence Samelson, associate director of the Center for Cancer Research at the National Cancer Institute, gave a high appraisal of this work: Shang et al by reporting a genome-wide CRISPRscreen in Jurkat T cells have performed an important service for the immunologycommunity. The lists of activating and inhibitory molecules that they havereported will be of great use to many investigators. This is an excellent studyand important contribution to those studying lymphocyte activation and signaltransduction in general.
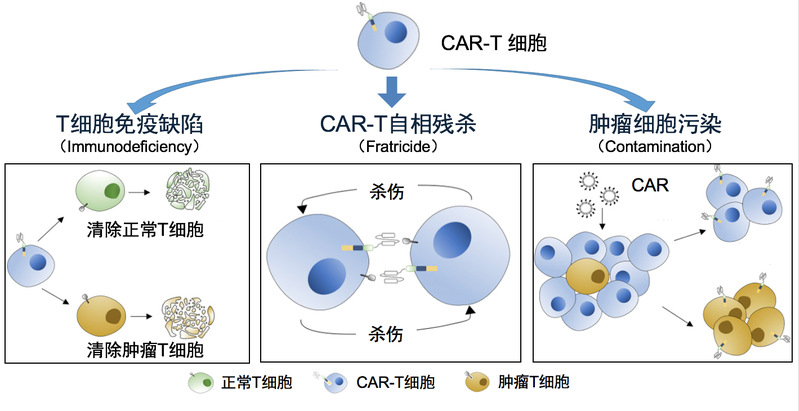
Shen Chi, Arthur Weiss, Haopeng Wang. A CRISPR-based toolbox for studying T cell signal transduction. BioMed Research. 2016. Wanjing Shang, Yong Jiang, Michael Boettcher, Kang Ding, Marianne Mollenauer, Zhongyi Liu, Xiaofeng Wen, Chang Liu, Piliang Hao, Suwen Zhao, Michael T McManus, Lai Wei, Arthur Weiss, Haopeng Wang.Genome-wide CRISPR screen identifies FAM49B as a key regulator of actin dynamics and T cell activation.PNAS. 2018 4. Establish CAR-T therapies for T-cell lymphoma (National Key R&D Program) CAR-T therapy has achieved remarkable results in the treatment of B cell-derived leukemia and lymphoma. But for T cell tumors that are also hemolymphatic tumors, CAR-T therapy has not been a suitable treatment plan. This is mainly due to the following fact: in vitro generated CAR-T cells, the patient's T cell tumor, and the T cells in the patient, all three of which are T lymphocytes by definition, and it is logically difficult to find a surface target to effectively distinguish these three types of cells (see the graph below). Our team cooperates with the first-line clinical team to develop a CAR-T therapy that can effectively treat T cell tumors. We expect to launch a clinical trial in early 2022.
Representative Publications (*First Author, # Corresponding Author)
MonographPatentFundingAwards
Research AchievementGroup Member and Photo
|