The prognosis of late stage cancer patients after traditional cancer treatments such as radiation, surgery and chemotherapy is always poor. Immunotherapy with antibodies targeting immune checkpoints represents a major breakthrough in cancer treatment. Some cases of patients previously consideredincurable are now able to be cured through immunotherapy. However, only a fraction of patients show durable response to immunotherapy, and the need for the discovery of biomarkers predicting the response to immunotherapy is urgent. The approval of mismatch repair deficiency as a tissue-agnostic immunotherapy biomarker by FDA has been selected as one of the ten scientific breakthroughs in 2017.
Cytidine deaminase APOBEC3B plays a key role in the mutation process of non-small cell lung cancer (NSCLC). APOBEC3B is also reported to be up-regulated and predicts bad prognosis in NSCLC. However, targeting APOBEC3B high NSCLC is still a big challenge. Recently a research team led by Assistant Professor Liu Xuesong of ShanghaiTech (lab homepage: https://liuxslab.netlify.com) has identified APOBEC3B expression and APOBEC mutation signature as immunotherapy response predictive biomarkers. Professor Liu’s team found that APOBEC3B up-regulation is significantly associated with immune gene expression, and that APOBEC3B expression positively correlates with known immunotherapy response biomarkers, including PD-L1 expression and T cell infiltration in NSCLC. Importantly, APOBEC mutational signature is specifically enriched in NSCLC patients having a durable clinical benefit after immunotherapy. Furthermore, APOBEC mutation count could be more accurate in predicting the response to immunotherapy than the total mutation count. Since APOBEC mutation signature is prevalent in several types of cancers including bladder, cervical, breast, head and neck, and lung cancer, it will be interesting to further investigate whether APOBEC mutation signature is another type of tissue-agnostic immunotherapy biomarker similar to the mismatch repair deficiency.
This work was published online in Oncogene on April 26, 2018. Graduate students Wang Shixiang and Jia Mingming are joint first authors and Dr. Liu Xuesong is the corresponding author. This work was supported by grants from the National Natural Science Foundation of China, Shanghai Municipal Science and Technology Commission and ShanghaiTech University.
Read More at: https://www.nature.com/articles/s41388-018-0245-9
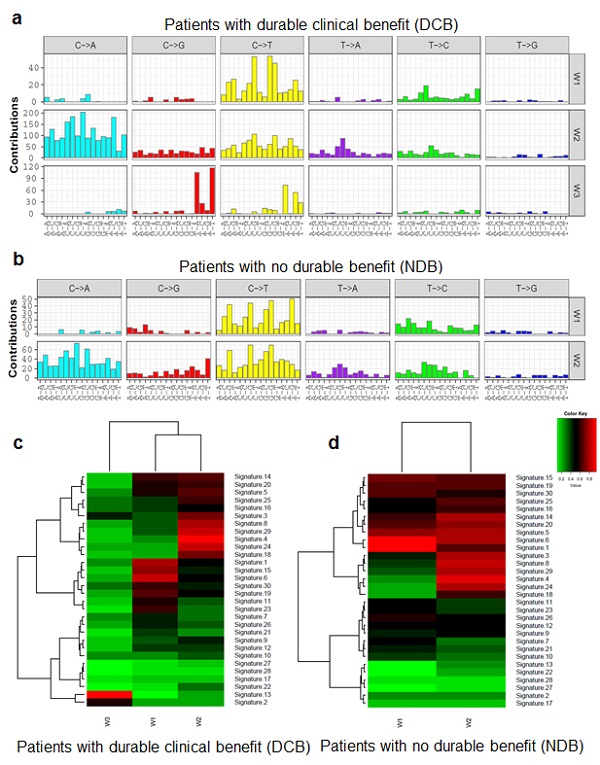
Figure 1:APOBEC mutational signature is specifically enriched in patients with durable clinical benefit but not in patients with no durable benefit.
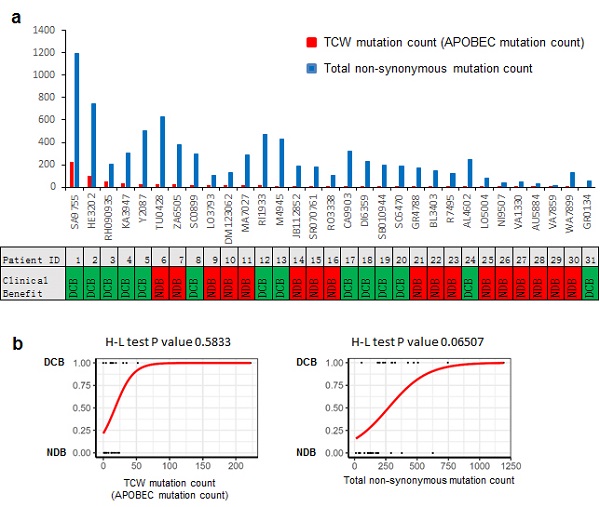
Figure 2:TCW mutation count (APOBEC mutation count) could be more accurate than the total non-synonymous mutation count, in terms of predicting PD-1 blockade immunotherapy clinical response.


