A team of scientists led by SLST’s Dr. Zhang Hui and CAS Shanghai Institute of Biochemistry and Cell Biology’s Dr. Zhou Bin, has discovered a new mechanism for neonatal heart regeneration. Their findings suggest that cellular senescence that occurs after heart injury plays an essential role in neonatal heart regeneration. Their work, entitled “CCN1-Induced Cellular Senescence Promotes Heart Regeneration” was published online in Circulation at May 21, 2019.
The researchers performed apical resection, a kind of heart injury, on neonatal hearts at postnatal day 1 and identified senescent cells in the injured regions in the following two weeks (Figure A). These senescent cells disappeared when the injured hearts were fully restored at postnatal day 21 (Figure A). The researchers found that clearing the senescent cells or inhibiting the formation of cellular senescence both impaired neonatal heart regeneration, which means that cellular senescence is required for neonatal heart regeneration. Subsequently, the researchers found that fibroblasts, one of the major cellular types in heart, were senescent post injury and Trp53 signaling pathway was required for fibroblast senescence and neonatal heart regeneration.
The researchers also found that CCN1, which is a kind of cellular matrix protein and is reported to induce fibroblast senescence in skin wound healing process, was highly expressed in cardiomyocytes after injury. Inhibition of the expression of CCN1 reduced the number of senescent cells and impaired the neonatal heart regeneration. In the study, the researchers concluded that cardiomyocytes secrete CCN1 to induce fibroblast senescence after injury and senescent fibroblasts promote neonatal heart regeneration by reducing fibrosis and secreting many kinds of proteins, such as interleukin-1a and interleukin-6, to promote cardiomyocyte proliferation (Figure B). Interestingly, the authors also found that CCN1 treatment also promoted cellular senescence, reduced cardiac fibrosis, and improved cardiac function in adult hearts after myocardial infarction. Together, cellular senescence is required for neonatal heart regeneration and CCN1 may be a potential drug to cure adult myocardial infarction.
This work was supported by grants from the Ministry of Science and Technology's key research program, the National Natural Science Foundation of China, the Shanghai Shuguang Program and the Startup Fund of ShanghaiTech University.
Read more at: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.119.039530
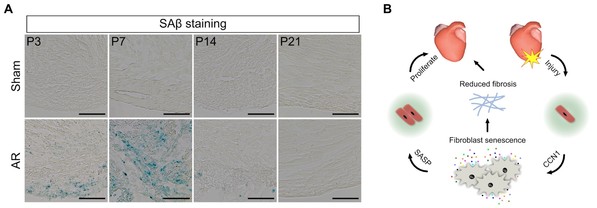
Cellular senescence promotes neonatal heart regeneration. A. Apical resection (AR) induced cellular senescence. AR was performed at postnatal day 1 (P1) and hearts were collected for senescence analysis at P3, P7, P14 and P21. B. Schematic diagram showing the mechanisms of neonatal heart regeneration regulated by fibroblast senescence.


