Research teams led by Professor Sun Bo from SLST and Professor Shen Bin from Nanjing Medical University have completed a high-resolution quantitative map of Cas9/sgRNA/DNA interactions (Figure 1). On November 13, 2019, their study was published as a research article entitled, “The post-PAM interaction of RNA-guided spCas9 with DNA dictates its target binding and dissociation, in the journal Science Advances.
CRISPR-Cas imparts bacteria and archaea with adaptive immunity against invasive viruses and plasmids. Due to the simplicity and specificity of programmable DNA recognition and cleavage by spCas9, it has been widely repurposed for genome editing, transcriptional perturbation and genomic imaging in various organism. Increasing its fidelity and minimizing off-target cleavage to ensure its efficient application in medicine and biology have been the focus of considerable research efforts.
These improvements demand a clear understanding of the interplay between spCas9 and DNA. Both biochemical and structural studies have shown that the nuclease activity of spCas9/sgRNA requires PAM recognition, DNA duplex bending and unwinding, almost complete RNA-DNA complementarity and subsequent subdomain conformational rearrangement. All these prerequisites are largely dictated by the critical and specific interactions of Cas9 with the target DNA. Thus, information about these interactions would aid in the understanding of the specificity of Cas9 in DNA targeting and cleavage and the development of Cas9 derivatives.
Using single-molecule optical trapping technique, Professor Sun and colleagues probed Cas9/sgRNA/DNA interactions along the DNA sequence and found two stable interactions flanking the protospacer adjacent motif (PAM). Unexpectedly, one of them is located approximately 14 base pairs downstream of the PAM (post-PAM interaction), which is beyond the apparent footprint of Cas9 on DNA. Loss or occupation of this interaction site on DNA impairs Cas9 binding and cleavage. Consistently, a downstream helicase could readily displace DNA-bound Cas9 by disrupting this relatively weak post-PAM interaction. This work identifies a critical interaction of Cas9 with DNA that dictates its binding and dissociation, suggesting a distinct strategy to modulate Cas9 activity.
This work was supported by the National Key R&D Program of China, Natural Science Foundation of Shanghai, ShanghaiTech University Startup funding, the National Science Fund for Excellent Young Scholars, the Science Foundation for Distinguished Young Scholars of Jiangsu Province, and the National Natural Science Foundation of China.
Read more at:https://advances.sciencemag.org/content/5/11/eaaw9807
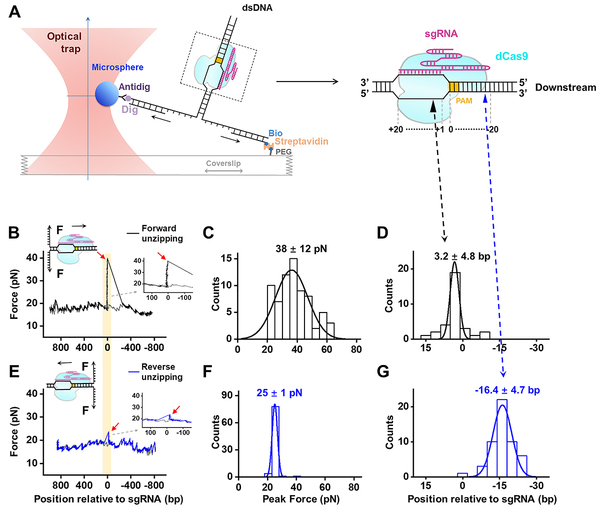
Figure 1. Experimental configuration for single-molecule unzipping experiments and spCas9-sgRNA-DNA interaction mapping.


