Professor Ji-Long Liu's group from the School of Life Science and Technology, ShanghaiTech University published a cover story entitled Combined inactivation of CTPS1 and ATR is synthetically lethal to MYC-overexpressing cancer cells in the March 15, 2022 issue of Cancer Research. This work demonstrates that selectively inhibiting CTPS1 combined with ATR inhibitor represents a promising strategy for the treatment of MYC-driven cancer in vitro and in vivo.
The cover image depicts the Battle of Chibi (meaning ‘Red Cliff’ in Chinese), one of the most famous battles in Chinese history. In the Battle of Chibi (208 AD), Sun Quan and Liu Bei allied the war of resistance against Cao Cao at the moment of the advance of strong enemies. They skillfully attacked with fire and finally defeated the strong with the weak. This war laid the foundation for the tripartite confrontation of the Three Kingdoms (220-265 AD).

Figure 1. A combination of two forces wins the battle. In the cover image, the fronts of two boats (in the shape of arrowheads) represent the Sun-Liu allies and the fire represents the battle. In this issue, Sun, Liu and colleagues target MYC-driven cancer with the combined inactivation of CTPS1 and ATR, similar to the Battle of Chibi (Concept: Ji-Long Liu; Illustration: Jia Du).
MYC has been established as an attractive cancer therapy target for decades. However, the lack of an obvious druggable domain and severe side effects render directly targeting MYC challenging. MYC supports cancer cell proliferation and survival through parallel induction of multiple key anabolic processes, such as the synthesis of nucleotides and proteins that must be highly coordinated to maintain anabolic balance. This has been proposed as a therapeutic vulnerability of MYC-driven cancer.
CTPS is the final, rate-limiting enzyme in the de novo synthesis of the nucleotide CTP, a precursor required for the synthesis of DNA, RNA, and phospholipids. In this study, Professor Liu and colleagues find that inhibition of CTPS selectively decreases cell viability and induces DNA replication stress in MYC-overexpressing human cells. This selective DNA replication stress is caused by MYC-driven rRNA synthesis, which makes CTP pool limiting for DNA replication.
DNA replication stress causes activation of the intra-S-phase checkpoint, mainly mediated by the ataxia telangiectasia-related kinase (ATR), to safeguard genome stability. Severe and prolonged replication stress results in DNA damage and cell death. They show that combined inhibition of CTPS and ATR induces DNA damage and apoptosis in MYC-overexpressing cells, dramatically decreasing tumor growth in vivo. This effect is independent of p53 and RAS status. This is very important as MYC and RAS signals are often activated together in tumor cells, and the p53 mutation is present in over 50% of cancers.
CTPS activity is encoded by two isoforms, CTPS1 and CTPS2; they were predicted to be functional redundant. Unexpectedly, Professor Liu’s team found that CTPS1, but not CTPS2, inhibition is required and sufficient to induce replication stress and consequent cell death when combined with ATR inhibition in MYC-dysregulated cancer cells. Further study showed that CTPS1 is the major transcription target of MYC and takes the main responsibility for supplying CTP to support MYC-elevated cancer cell proliferation and survival.
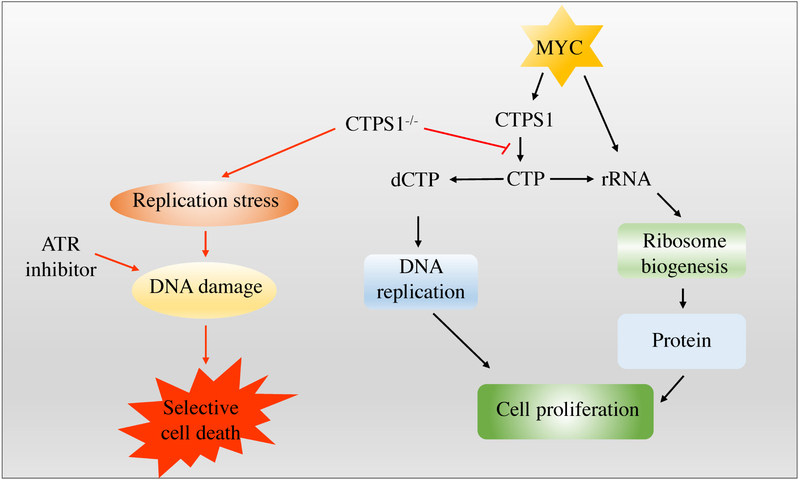
Figure 2. Combined inhibition of CTPS1 and ATR induces synthetic lethality in MYC-overexpressing cells. MYC-overexpressing cells are dependent on CTPS1 for DNA replication and rRNA synthesis. Inhibiting CTPS1 induces replication stress and consequent apoptosis when combined with ATR inhibitor in MYC-overexpressing cells.
These results would be of interest to the scientific community because CTPS1 deficiency causes no significant clinical consequences. Thus, targeting CTPS1 combined with ATR inhibitor would be highly specific in killing MYC-overexpressing cancer cells without additional side-effects given the redundancy of CTPS2 activity in normal tissues.
In the same issue, a commentary article highlighted the significance of the study by Professor Liu’s team, “Sun and colleagues found a unique function of the rate-limiting nucleotide synthesis enzyme CTP synthase 1 (CTPS1) in the survival of MYC-driven cancer cells. They further identified a novel synthetic lethal strategy to combat MYC-driven cancers...... These findings open novel therapeutic avenues for targeting the traditionally ‘undruggable’ MYC-driven cancers, which represent one of the highest unmet clinical needs in cancer”.
Link to Professor Liu’s article: https://aacrjournals.org/cancerres/article/82/6/1013/682062/Combined-Inactivation-of-CTPS1-and-ATR-Is
Link to the March 15, 2022 issue of Cancer Research: https://aacrjournals.org/cancerres/issue/82/6


