On July 28, Li Na et al. from Zhu Lab from the School of Life Science and Technology published research titled A sphingolipid-mTORC1 nutrient-sensing pathway regulates animal development by an intestinal peroxisome relocation dependent gut-brain crosstalk in the journal Cell Reports. Using Caenorhabditis elegans as the model organism, this study found that the subcellular localization of peroxisomes in intestinal cells and the hormonal signals they produce could mediate the inter-tissue regulation of animal development via a sphingolipid/mTORC1 nutrient-sensing pathways.
How animals adjust their developmental fate to adapt to the environment by sensing the nutrition availability has been a long-standing problem that has fascinated biologists since the era of Darwin. In recent years, a series of seminal works in this field have found that the mTORC1 pathway, which is the central hub of nutrient sensing, can convert nutrient and growth signals in the environment into growth signals in cells. However, the vast majority of studies to date have only been performed in mammalian tissue-cultured cells and have not been validated in vivo under physiological conditions. Moreover, perception of nutritional availability is often restricted to certain tissues. How individual tissues transmit nutrient signals across the body and coordinate overall developmental fate remains poorly understood.
Glucosylceramide is a special lipid that structurally integrates amino acids, fatty acids, and carbohydrates, and is very conserved in humans and other animals. Lack of glucosylceramide leads to early developmental arrest and death in humans and nematodes, fruit flies, and mice, but its molecular mechanism and physiological significance have remained unknown. In 2013, an article published in eLife by Zhu Huanhu and others from Professor Dr. Min Han’s Lab at the University of Colorado found that glucosylceramide, as an in vivo metabolite of monomethyl branched-chain fatty acids, can activate the nutritional sensing hub mTORC1 pathway to regulates C. elegans development. Recently, Mengnan Zhu and others from the Zhu Lab discovered that the main physiological purpose of the above-mentioned developmental signaling pathway is actually to sense the overall amino acid in food and correspondingly regulate the developmental fate of C. elegans; and this regulatory mechanism is also conserved in mammalian cells. However, similar to other studies in the mTORC1 field described above, how this gut-directed glucosylceramide/mTORC1 pathway coordinates whole-body development in animals remains a mystery.
To address these problems, the researchers first discovered a gene mutation encoding the peroxisomal membrane protein PRX-11, which is conserved from yeast to humans, through a large-scale random mutagenesis screening, which could make glucosylceramide-deficient C. elegans bypass the developmental arrest and complete its development. Further research found that mutations of other key peroxisomal proteins such as PRX-5, PRX-3, and PRX-1 can cause similar effects. These data suggest that peroxisome activity actually plays a key role in the developmental arrest caused by glucosylceramide deficiency. By tissue-specific rescue experiment, the researchers found that gut and muscle peroxisomal activity of PRX-11 is critical for glucosylceramide-mediated C. elegans development. Further studies found that the developmental recovery effect of PRX-11 mutant on glucosylceramide-deficient animals was not dependent on mTORC1, and PRX-11 mutant could rescue the developmental diapause caused by mTORC1 deficiency, indicating that PRX-11 acts on the downstream of glucosylceramide/mTORC1 to mediate the developmental regulation. This finding has also become the core question of the entire study: which function of the peroxisome, a seemingly inconspicuous organelle, is the most important in mediating nutritional perception and developmental regulation?
The researchers surprisingly found that in the absence of nutrients or glucosylceramide, or downregulation of mTORC1 activity, peroxisomes were no longer evenly distributed in C. elegans enterocytes, but accumulated in the apical membrane, a region close to C. elegans’ gut lumen, and the PRX-11 mutation was able to reverse the aggregation of peroxisomes and the corresponding diapause phenotype. Further studies found that the intestinal apical membrane aggregation of peroxisomes is dependent on the kinesin 1/microtubule trafficking machinery. Disrupting the kinesin protein Kinesin 1 blocked the intestinal apical membrane aggregation of peroxisomes as well as the developmental arrest in glucosylceramide deficiency animals. These data suggest that the aggregation of peroxisomes was the direct cause of developmental diapause. Further research found that these peroxisomes accumulated in the intestinal apical membrane synthesize and secrete a class of small-molecule glycolipid hormone-Ascaroside, which is derived from the peroxisomal β-oxidation. Those ascarosides hormones further acted on its receptor DAF-38 on the cilia of chemosensory neurons on the C. elegans head, and the downstream nuclear hormone receptor DAF-12, to inhibit development in glucosylceramide-deficient animals. Therefore, the intestinal tissue can regulate the developmental process by secreting small molecule hormones that act on the central nervous system according to its own nutritional sensing signals.
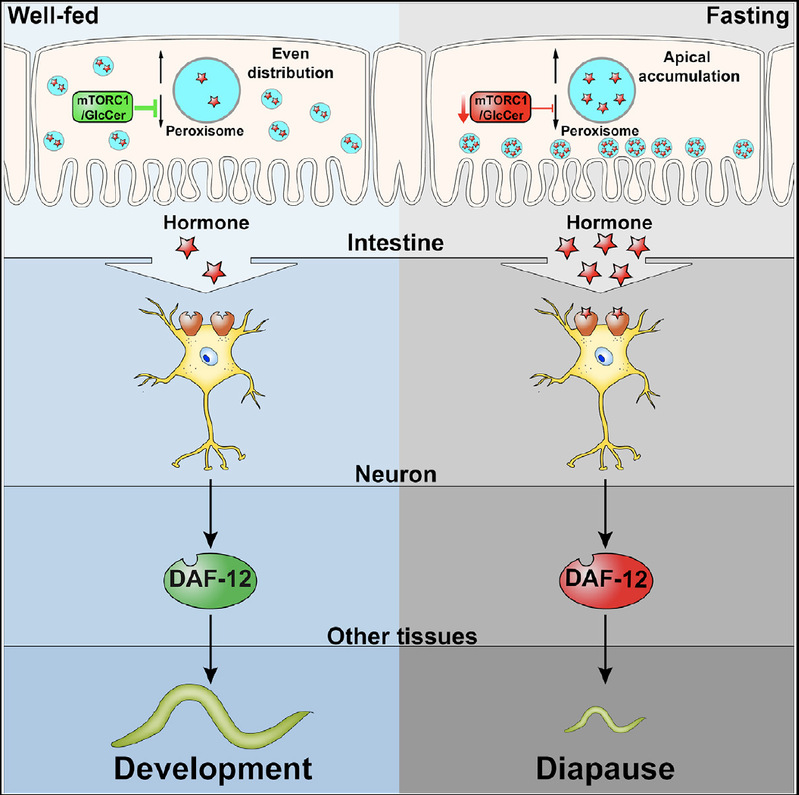
Overall, Li et al. found a gut-brain axis pathway in C. elegans that senses external nutrients and regulates development through sphingolipid metabolites, nutrient sensing pathways, the organelle subcellular localization, and hormone secretion. At the same time, the researchers also found that the intracellular distribution of peroxisomes regulated by the nutrient environment is conserved in mammalian cells, which also lays the foundation for the future understanding of similar processes in higher organisms.
Finally, what is the physiological significance of this complex model of developmental regulation? The researchers found that although the dual deficiency of glucosylceramide and peroxisomes had limited effects on C. elegans grown under abundant nutrient conditions, it significantly decreased C. elegans tolerance to starvation. From this, the researchers raised a bold hypothesis that glucosylceramide and peroxisomes may act as positive and negative regulators in animal development respectively. Just like the accelerator and the brakes of a car, the two work together to ensure that the car (animals) can adjust its (developmental) speed according to the road (environmental nutrition) conditions, thus being preserved in the evolution. This may also help explain why, in the evolution, glycosphingolipids and peroxisomes are not necessary for unicellular eukaryotic growth, but are necessary for the development of multicellular organisms.
Li Na, a 2018 doctoral student, and Hua Beilei, a 2022 doctoral student, from the Zhu lab, School of Life Science and Technology, ShanghaiTech University are the co-first authors of the paper, master student Chen Qing (graduated), Research Assistant Professor Dr. Fukang Teng, doctoral student Ruan Meiyu; Zhu Mengnan (graduated), Zhang Li, Professor Min Zhuang from the School of Life Sciences of ShanghaiTech University and her doctoral students Huo Yinbo, and Liu Hongqin; and Professor Shen Huali from Fudan University also participated in the research. The project has been funded by the General Program of the National Natural Science Foundation of China, the key research and development projects of the Ministry of Science and Technology, the introduction of overseas high-level talents, the Science and Technology Commission of Shanghai Municipality, and the ShanghaiTech University.


