In the early 1970s, a milky white drug called propofol was discovered to have a stable sedative effect, thus vividly referred as anesthetic milk. This drug is privileged to avoid the side effects caused by other intravenous anesthetics on the human body, and therefore has been widely applied for both general anesthesia and short surgical procedures since its official clinical use in 1986. Interestingly, clinical practice has repeatedly shown that the intravenous administration of propofol not only induces hypnosis, sedation, and amnesia, but also often generates euphoric moods and lasting relaxation. Actually, the recreational use of propofol has been consistently reported to produce a well-rested feeling and euphoric state; yet, the neural mechanisms underlying such pleasant effects remain unclear.
On March 13th, Prof. Ji Hu from the School of Life Science and Technology of ShanghaiTech University and his collaborators published a research paper entitled Propofol exerts anti-anhedonia effects via inhibiting dopamine transporter in Neuron, exploring the molecular targets and neural circuit mechanisms of the pleasant feelings produced by propofol and discovering a novel therapeutic application for this old drug in the treatment of depression.
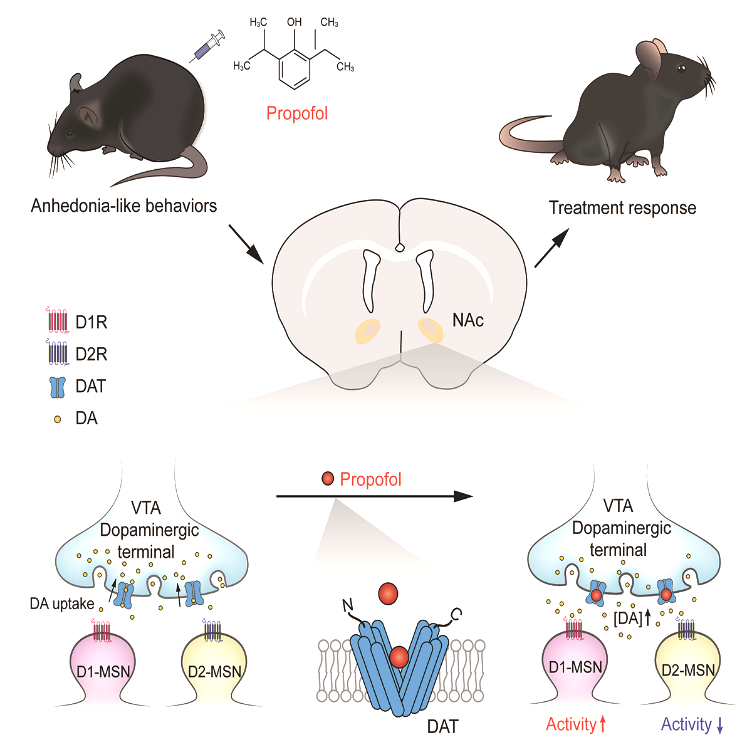
This study systematically screened the effects of propofol on various neurotransmitter or neuromodulator systems in the brain. Employing molecular docking, molecular dynamics simulation, and other biochemical and cellular assays, the authors firstly reported that propofol specifically binds to the dopamine transporters (DATs), a key arbiter in regulating extracellular dopamine homeostasis. Interestingly, the binding model of propofol to DAT differs from that of other known drugs, such as cocaine, which is consistent with the clinical observation that propofol is less addictive. Secondly, by using positron emission tomography imaging and fiber photometry in freely behaving mice, the authors show that a single dose of propofol could efficiently block the reuptake of dopamine and thus produce a sustained dopamine release in the nucleus accumbens, a critical reward center in the brain. During this process, dopamine receptor 1 (D1)-expressing neurons show increased activity, while the activity of dopamine receptor 2 (D2)-expressing neurons decreases, thus participating in the formation of pleasure.
Next, the authors further examined whether the above actions of propofol can be applied to treat the anhedonia, one of the core symptoms for several major psychiatric disorders, especially major depressive disorder. They established the chronic restraint stress model to mimic the daily psychological stress of depressed patients and found that a single dose of propofol can effectively reverse the anhedonia phenotype of the chronically stressed animals 4 h after i.p. administration, with the beneficial effects lasting for at least 3 days. Finally, using the chemogenetic manipulations, the authors revealed that inhibiting the activity of D1 expressing neurons in the nucleus accumbens abolished the anti-anhedonia effect of propofol. These findings suggest that propofol can elevate dopamine levels and activate D1 expressing neurons through selectively binding and inhibiting the DAT activity. Elevation of dopamine signaling by propofol will then lead to rapid and lasting relief of anhedonia.
Taken together, this study reported the effect of propofol on accumbal dopamine through its direct binding to DAT, supporting the non-anesthetic use of the anesthetic milk in relieving anhedonia associated with several neuropsychiatric diseases. Ongoing studies from this group have included the structural modification of propofol and analogs to further screen new antidepressant drugs with better efficacy and lower addiction risk.
Dr. Xiao-Na Zhu, the assistant researcher in Ji Hu's group, Jie Li, PhD student in Shanghai Mental Health Center, and Gao-Lin Qiu, PhD student in Anhui Medical University are the co-first authors of the paper. Ji Hu, Ti-Fei Yuan and Tao Xu are co-corresponding authors and supervised the study.
Paper links: https://www.cell.com/neuron/fulltext/S0896-6273(23)00121-6


